Is my Product a Medical Device? Slide 1 Hello, my name is Commander Kimberly Piermatteo of the United States Public Health Servi
Guidance for Industry: Regulatory Framework for Substances Intended for Use in Human Food or Animal Food on the Basis of the Gen
DIVISION ll—HEALTH PROVISIONS TITLE I—PUBLIC HEALTH Subtitle A—National Disaster Medical System Subtitle B—Synthetic Nic
FDA and Industry Procedures for Section 513(g) Requests for Information under the Federal Food, Drug, and Cosmetic Act - Guidanc

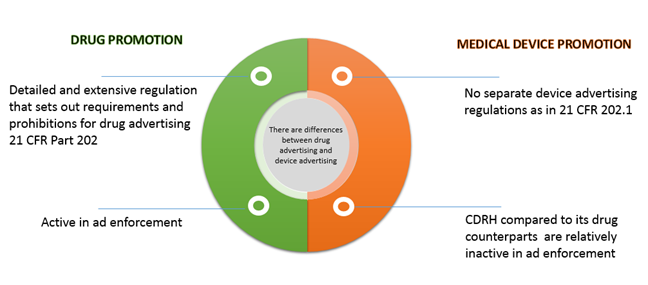
Book 7: 2023 Selected Laws/Regulations/Guidance on Drug Marketing, Adv – Clinical Research Resources, LLC
Referencing the Definition of “Device” in the Federal Food, Drug, and Cosmetic Act in Guidance, Regulatory Documents, Commun